Abstract
Introduction : Acute graft-versus-host disease (aGVHD) remains a major barrier to the success of allogeneic hematopoietic stem cell transplantation (HSCT). Mechanistically, aGVHD depends on the trafficking of donor conventional T cells (Tcons) into host secondary lymphoid tissues (SLT) where they are activated by host antigens. Previously our group demonstrated that donor Tcons deficient in the lymphoid trafficking receptor CC-Chemokine Receptor 7 (CCR7) generated attenuated aGVHD responses but retained their graft-versus-leukemia (GVL) potential (Coghill JM et al. Blood. 2010; 115(23): 4914-22). This suggested that the receptor might be targeted therapeutically. Since there were no drugs affecting the CCR7 axis, we undertook a high throughput screen (HTS) of over 200,000 compounds to identify small molecule antagonists of the receptor. This effort demonstrated that cosalane, a compound originally developed as a novel HIV therapeutic but with additional activity against numerous other enveloped viruses including cytomegalovirus and herpes simplex virus, also possessed an intrinsic ability to block human and murine CCR7 signaling in response to both of its ligands. As a result, we examined the ability of cosalane to modulate aGVHD in vivo in mouse HSCT models.
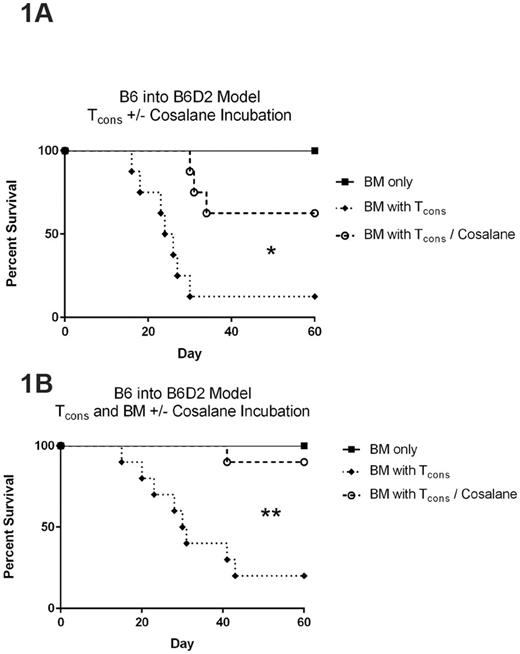
Results : Cosalane did not substantially alter aGVHD outcomes when dosed intravenously post-transplant to murine recipients in a standard C57BL/6 "B6" (H-2b) into C57BL/6-DBA2 F1 "B6D2" (H-2bxd) haplotype matched HSCT model. However, when donor Tcons were incubated with cosalane in vitro for one hour prior to transplant, aGVHD was significantly attenuated and survival was prolonged compared to cells incubated with DMSO vehicle (Figure 1A, *P=0.004 for curve comparison between BM with Tcons and BM with Tcons/cosalane groups by log rank test). Cosalane exposure did not appear to result in any cytotoxicity, and did not affect Tcon activation and expansion in vitro in response to CD3 stimulation in the presence of supplemental IL-2. Furthermore, when the entire stem cell product (bone marrow cells plus Tcons) was incubated with cosalane prior to transplant, myeloid engraftment was unaffected and survival times were once again improved with no evidence of bone marrow aplasia (Figure 1B, **P=0.001 for curve comparison by log rank test).
While cosalane was originally identified as an inhibitor of CCR7, its effects on donor Tcon trafficking into host lymphoid sites post-transplant were surprisingly modest. No major differences in donor Tcon accumulation within the host inguinal lymph nodes, mesenteric lymph nodes, or spleen were noted on either transplant day +7 or day +14. A strong trend for reduced Tcon numbers was noted, however, within the host colon and liver by the second week post-transplant, implying an additional mechanism of action beyond its activity against CCR7.
Cosalane's effects did not appear to be strain dependent, as survival times were also improved in a completely MHC mismatched B6 into BALB/c (H-2d) model system, although to a lesser extent. In addition, cosalane pretreatment of human peripheral blood mononuclear cells resulted in prolonged survival times in a mouse xenogeneic aGVHD transplant model. We went on to examine cosalane's effects on beneficial GVL effects using a well described luciferase transduced p815 (H-2d) murine mastocytoma model. Here survival times were prolonged in B6D2 mice receiving cosalane treated B6 Tcons/tumor versus those receiving DMSO treated B6 Tcons/tumor (who all succumbed to aGVHD) and those receiving bone marrow alone/tumor (who all died from malignancy). We have found cosalane's effects on aGVHD to be dose dependent in a B6 into B6D2 system (treatment range 13uM-39uM), and additional work is ongoing to optimize its administration so as to maximize anti-inflammatory effects while sparing anti-tumor immunity.
Conclusions : The anti-viral compound cosalane is able to attenuate aGVHD in multiple allogeneic and xenogeneic model systems after a brief incubation with the donor Tcon and/or bone marrow product in vitro prior to transplant. Although identified in our HTS as a CCR7 antagonist, its effects on lymphoid trafficking after HSCT have thus far been limited, and additional mechanistic studies are currently ongoing. Given its known anti-viral effects against numerous pathogens common to the HSCT setting, we are also exploring its anti-infective properties in murine model systems.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal